Here, Dr SUN Libo explores a new, more efficient method of converting carbon dioxide into other useful materials. This is a preview from CARES’ latest biannual report, which will be available soon.
The problem of climate change is currently attracting widespread attention due to its negative impact on people around the world. Disasters like glacial melting, sea level rise, changing ecosystems, increased droughts and floods and deadly heat waves are no longer hypothetical ideas but real threats to society. It is widely accepted that the emission of greenhouse gases like carbon dioxide (CO₂) from the burning of fossil fuels, agriculture and deforestation, mainly initiated by humans, is the main culprit of climate change. An observable temperature increase has accompanied these fossil fuel emissions and the concentration of CO₂ in the atmosphere has increased from 350 ppm (the upper safety limit) in 1990 to around 400 ppm today. Furthermore, the depletion of the non-renewable fossil fuels due to the world’s fast economic development and population growth is contributing to an energy crisis while rapidly increasing CO₂ emissions into the atmosphere. It is time to develop new paths to obtain the world’s required energy, while at the same time reducing our harmful emissions.
Numerous methods have been devised in an effort to solve this problem, including the development of novel, clean and renewable energies (e.g. wind, solar and tide) and ways to decrease the concentration of atmospheric CO₂. Harnessing these alternative energies can partly replace the use of fossil fuels. In addition, CO₂ capture and sequestration (CCS) techniques are being explored to decrease the amount of CO₂ released into the atmosphere. However, fossil fuel is still irreplaceable and makes up more than 80% of total energy consumed per year, while CCS techniques are imperfect solutions due to their expensive price, high energy consumption and the required storage space.
Another solution is to convert CO₂ into other useful chemicals. A great many techniques to implement this have been explored, including electrochemical and biological options. Among the current methods being developed, electrochemical CO₂ reduction is the most attractive due to its simple operating conditions and the diversity of chemicals it can produce. This process uses electricity to convert CO₂ to a number of other chemicals and can be an energy-saving process. Electrochemical CO₂ reduction uses electricity applied across electrodes (one positive and one negative) to drive a chemical reaction that wouldn’t otherwise occur. The products of these reactions usually include organic feedstocks like formic acid (CH₂O₂), carbon monoxide (CO) and methanol (CH3OH), and other hydrocarbons like methane (CH4), ethylene (C2H4) and ethane (C2H6). Electrochemical CO₂ reduction can both reduce CO₂ emissions into the environment and produce useful fuel sources.
The latest research has shown that in a standard electrochemical setup, a small change in the relative electrical potential of the solid and liquid phases can give a greatly improved reaction rate, requiring a lower amount of energy to get started, when compared with other methods mentioned above. Using this reaction, researchers have found that metals like indium, tin and lead tend to produce formic acid, while silver, gold, palladium and zinc may produce carbon monoxide. Copper is unique as it can produce a series of different products, including C1-C2 hydrocarbons with different selectivity. Despite the versatility and low energy requirements of electrochemical CO₂ reduction, the technique is not yet perfect. We are still searching for excellent catalysts that are economical, have a low activation energy to initiate the reaction, a high selectivity to designated product, longer stability and a high current density during the process.
A possible candidate for this is single-atom catalysts, which have drawn much interest over the last few years. In such catalysts, the metal active sites are evenly dispersed onto the substrates, which means a highly efficient use of metal to the atomic level. Standard electrochemical reduction techniques incorporate materials of varying surface orientations, which can affect the amount of organic feedstock produced. In single-atom catalysts, however, the even dispersion of atoms on the substrate gives a fast and highly uniform production rate. Some factors need to be considered when fabricating such catalysts:
One, all the atoms should be exposed to the surface of the substrate, which means the electrochemical surface area should be large enough to react with electrolytes directly, and diminish the mass transport to the inside layers of catalysts. Two, the substrate should be conductive, helping to make the charge transfer more efficient, so carbon-based, or element-doped carbon can be an ideal substrate. Three, the stability and durability of catalysts needs to be considered, as a large overpotential (excess energy) may induce the single atoms to aggregate during the process. Four, the mass loading should be as large as possible while keeping a highly dispersed structure, and thus a higher current density. Finally and most importantly, to understand the reaction mechanism of such single-atom catalysts, there is no doubt that the synergistic effects between the substrate and the metal atoms used may contribute to performance and efficiency.
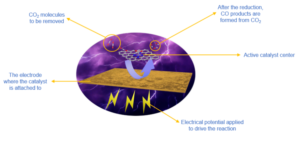
Based on the points mentioned above, we aim to develop single-atom catalysts with uniform and large loading efficiency, high conductivity and better stability and durability. We have therefore selected a nitrogen-doped carbon substrate to prepare single-atom metals with transition metals (such as iron, cobalt, nickel and copper) to form metal-nitrogen-carbon catalysts. Such catalysts can better utilise atoms, exhibit higher selectivity during the tests, reduce wasted energy and increase stability. One catalyst that we are currently investigating could exhibit higher selectivity towards carbon monoxide, above 90%, in the testing range of -0.65 to -1.05 V versus the standard reversible hydrogen electrode.
Future research promises continual improvements to these catalysts. Eventually, the technology will have developed to the point where there can be large-scale application in areas such as the petroleum refinery industry. Cleaning up and transforming some of the waste produced by refineries through electrochemical reduction is one of many ways in which we can reduce our atmospheric carbon dioxide levels and mitigate the damaging effects of climate change.
Dr Sun Libo received his PhD thesis under the supervision of Prof. YU Jihong in State Key Laboratory of Inorganic Synthesis & Preparative Chemistry, Jilin University, China. His PhD research focused on the synthesis of porous materials, including mesoporous and microporous materials, metal-organic frameworks and porous organic polymers. For his current Cambridge CARES project, his interest has turned to electrochemical areas and he is now focused on CO2 reduction and oxygen evolution, with complex or single-atom catalysts.


