The following article by CARES Research Fellow Dr Alexandr Khudorozhkov is taken from the 12th CARES Research Report.
In general, separation is the process of dividing a mixture into constituents or distinct elements.
In chemistry and the chemical industry, separation of mainly gas and liquid mixtures is a common process, used primarily to achieve two results: obtaining a pure compound and increasing the yield of the target product or products. Almost all separation techniques include a combination of physical (gravitation, electromagnetic field, physical adsorption, evaporation/distillation) and chemical (chemisorption, specific chemical reactions) processes.
Separation of a gas mixture containing products of carbon dioxide reduction such as carbon dioxide, hydrogen and hydrocarbons can effectively be done via “molecular sieving”, when compounds like zeolites with particular pore size and specific volume may be used as sieves to separate molecules of different sizes from each other (Figure 1).
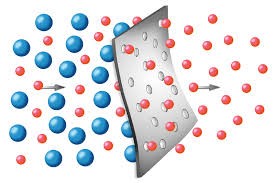
Fig. 1: Molecular sieving effect.
Another promising material for the separation of gas mixtures is metal-organic frameworks (MOFs). The typical MOF molecule (Figure 2) is a central ion (usually metal) surrounded by ligands (organic compounds). It forms “pores” that are used as molecular sieves. The separation efficiency of MOFs can also be enhanced with a chemical interaction between target molecules of a gas mixture and metal ion or ligands of the MOF occur.
Changing the synthesis procedure of MOFs and zeolites can help to obtain structures with different pores and central ions, which may lead to a significant increase of overall performance through better separation efficiency, stability and selectivity.
Besides the utilisation of MOFs and zeolites in the above-mentioned process of the separation of products of carbon dioxide reduction, one of the most promising ways of using of these materials is the refinement of crude oil, which consists of a mixture of hydrocarbons with varying lengths of carbon chain. Separation is necessary to obtain light (petrol, gasoline, naphtha) and heavy (diesel, kerosene, tar) oil fractions with consequent refinement. The most widespread up-to-date technique used for the separation of crude oil is fractional distillation – components are being separated due to different boiling temperatures and then condensed. Although this process is effective, it is highly energetically unfavourable. Utilisation of specific MOFs and zeolites in crude oil separation as well as in consequent petroleum refining and naphtha cracking may significantly decrease operation costs while providing the same effectiveness and selectivity.
Production of high-purity oxygen and hydrogen for fuel cells and other applications may also be done via separation. Some MOFs and zeolites may efficiently separate air (containing 78% nitrogen and 21% oxygen) to obtain oxygen. Varying the structural properties of these compounds may well help to successfully utilise them in the production of pure hydrogen via its separation from carbon monoxide (CO) in processes of natural gas (containing mainly methane, CH4) steam reforming and partial oxidation:
CH4 + H2O = CO + 3H2,
2CH4 + O2 = 2CO + 4H2.
More in-depth investigation of structural and physical-chemical properties of zeolites and MOFs may lead to the development of new and promising materials which could be used in many important industrial separation processes.
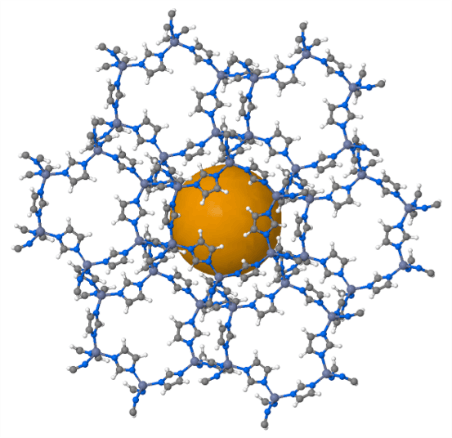
Fig. 2: Structure of a metal-organic framework (MOF).
Alexandr graduated from Novosibirsk State University in 2011 with a Specialist degree in “Chemistry”. He obtained a PhD degree in “Chemical kinetics and catalysis” in 2018 and worked in the Boreskov Institute of
Catalysis, Novosibirsk, Russia from 2008 until 2018. He joined CARES at the end of 2018. Alexandr’s research interests include inorganic chemistry, coordination chemistry, physical chemistry, heterogeneous catalysis, chemical engineering and the programming language Java.


