An image is worth a thousand words and in this case, best represents our achievements from the various scientific programmes we have participated in since the centre’s inception. The scientific images were compiled as part of CARES’ 10th-anniversary celebrations in 2023.
1. A different type of nano(crystal)
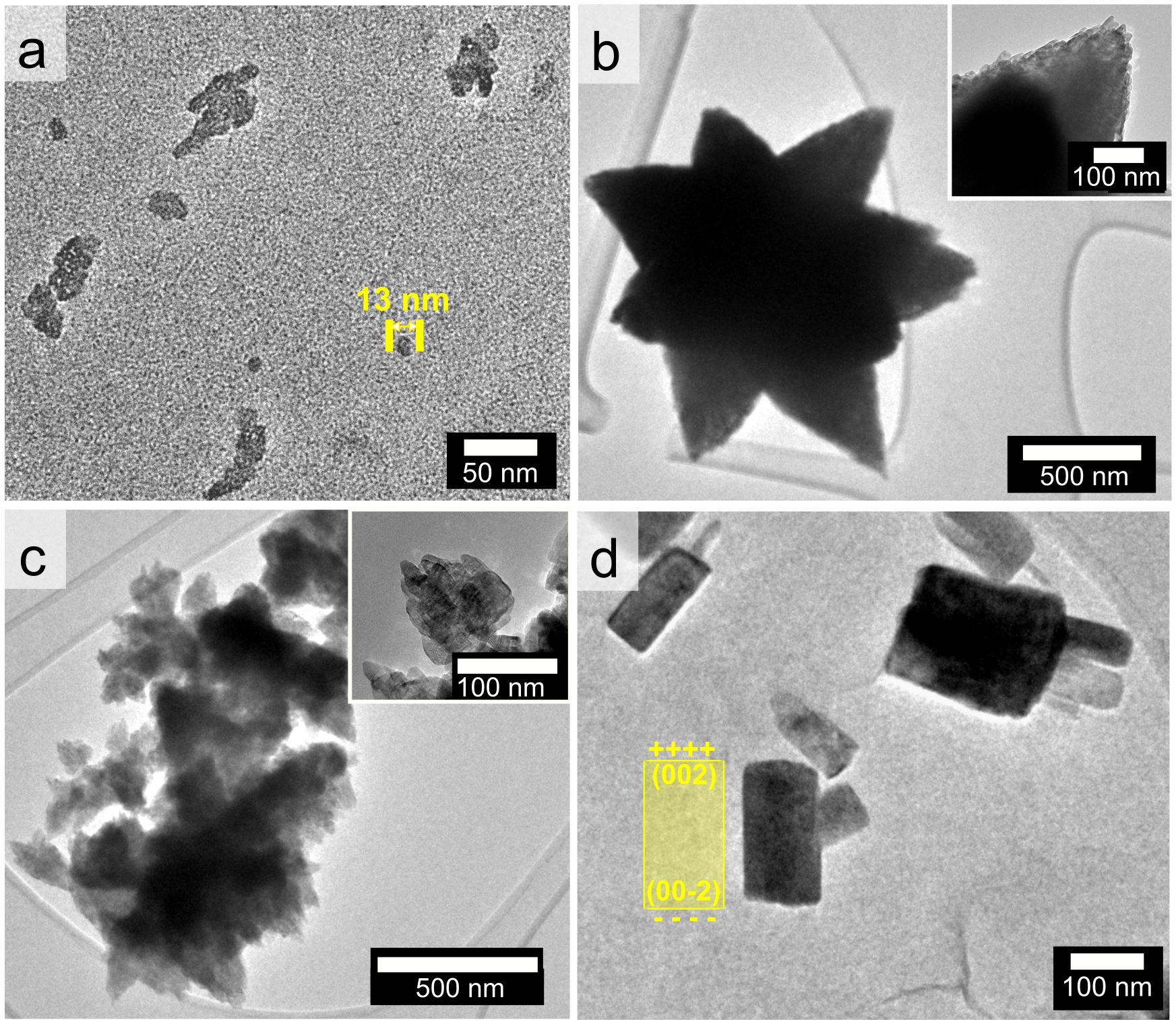
Zinc oxide nanostructures formed with annular microreactor technology. These structures are formed from smaller nanocrystals, which self-assemble to form a variety of nanostructures.
Why is this interesting? Nanostructured zinc oxides are incredibly useful in antimicrobial applications, catalysis, energy storage and medicine. However, it is difficult to precisely control how different nanostructures form at large scales. In this study, CARES researchers from the C4T (Centre for Carbon Reduction in Chemical Technologies) programme created these nanostructured zinc oxide stars, flakes, and rods with high precision at kilogram scales using annular microreactor technology. The team used machine learning to discover which shapes were more antimicrobial than others.
What happened since producing this image? This finding helped to prove that the combination of annular microreactor technology and machine learning can rapidly churn out high-performance nanomaterials at industry scales. This ultimately guided the Accelerated Manufacturing Platform for Engineered Nanomaterials (AMPLE) project which received NRF’s Central Gap Fund in June 2022. AMPLE formed a spinout called Accelerated Materials to act as a commercialisation engine for all technologies generated from the project.
Acknowledgements Dr Nicholas Jose, Dr Mikhail Kovalev, Umamaheswari Kencha, and Prof Alexei Lapkin were involved in the creation of this image and the core team to start AMPLE. Financial support came from NRF* and the Singapore MIT Alliance for Research and Technology for a SMART Innovation Centre Innovate Grant for the proof-of-concept project.
2. A computer-generated carbon foam

Nanoscale structure of disordered carbon generated by computer simulation. The sheets are single-atom layers of carbon called graphene. They are knotted together and curved. The curvature iscoloured (red is saddle-shaped, blue is bowl-shaped, and green is flat).
Why is this interesting? Scientists were struggling to determine the exact nanostructure of disordered carbon. The structure has layers of carbon like graphite in a pencil, but these layers are tangled in a mess. So CARES researchers simulated this tangle of atoms to discover how they’re connected. They found that the atoms come together in warped, curved sheets that connect in 3D to resemble carbon foam. These results
help us understand and engineer new carbon materials for applications in supercapacitors, carbon fibres and high-temperature ceramics.
What happened since producing this image? Dr Jacob Martin, the first author of this research, continues to untangle carbon structures in his new position as Forrest Fellow at Curtin University. His recent research has discovered insights that could have significant cost savings when it comes to the industrial production of graphite. Graphite is a critical component of lithium-ion batteries.
Acknowledgements Dr Jacob Martin and Prof Markus Kraft from CARES collaborated with Carla de Tomas, Irene Suarez-Martinez, and Nigel Marks from Curtin University for this research. They performed large-scale simulations using Australia’s Pawsey supercomputer to self-assemble the largest and most accurate networks of disordered 3D graphene networks to date. Financial support came from NRF* and the
Australian Research Council (No. FT140100191) as well as helpful discussions on differential topology with Dr Clive Wells, Hughes Hall, Cambridge
3. Imagining future ships of the high seas
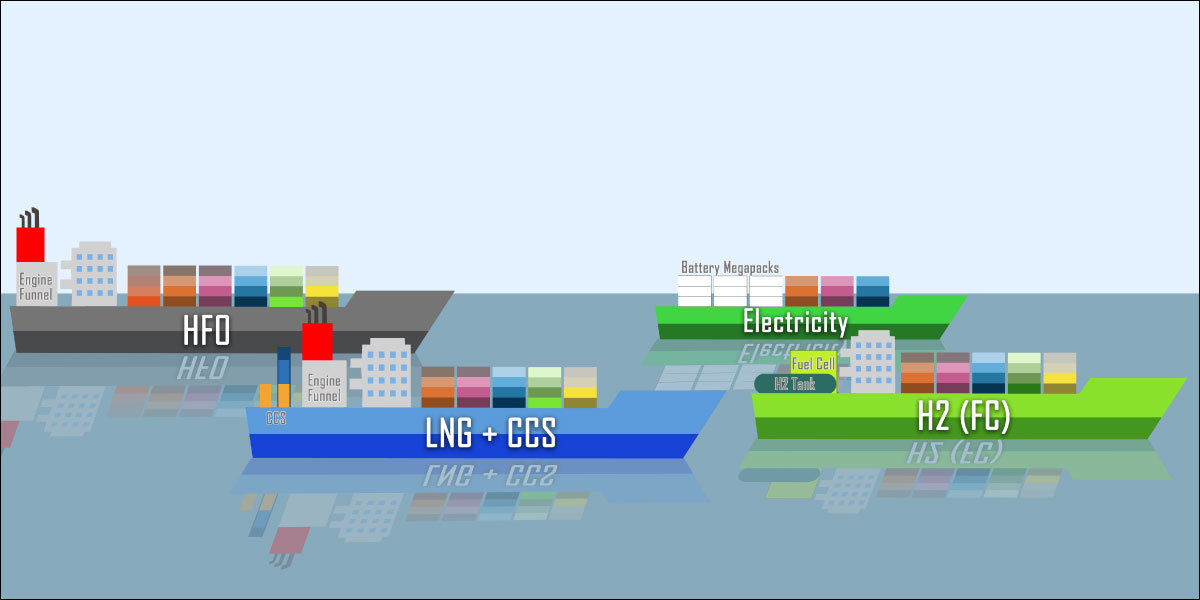
CARES research is the foundation of an interactive calculator that estimates emissions if alternative fuels are used in shipping. Users can input parameters like ship type, speed, deadweight, fuel type, and power rating to calculate the performance of current and future ships.
Why is this interesting? This online calculator is the result of many in-depth studies on shipping decarbonisation. It can reveal the potential cost, energy, and carbon saved by transitioning to alternative maritime fuels. There is no single solution to decarbonise the shipping industry. So, quantifying many parameters lets users compare and optimise the performance of different ships powered by various alternative fuels. With this tool, academics, engineers, shipowners, project managers and policymakers can make informed decisions to meet the 2050 maritime decarbonisation goals.
What happened since producing this image? CARES researchers are now focusing on refining the database and developing rigorous thermodynamic models for ships powered by methanol and biofuels. The calculator website has been actively promoted and shared to foster discussion among professionals within the shipping industry; the calculator continues to be refined with industry feedback.
Acknowledgements Dr Li Chin Law and Prof Epaminondas Mastorakos led this research with additional support from Jessie Smith, Dr Savvas Gkantonas, and the Registro Italiano Navale (RINA). Financial support came from NRF*.
4. Crystalline fields forever
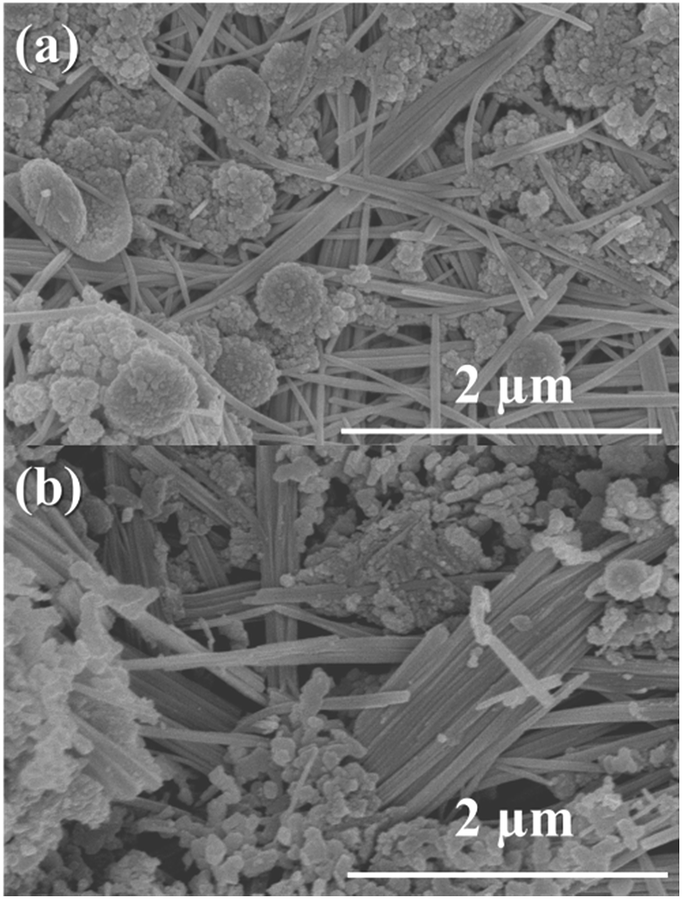
Two crystal structures are observed during the preparation of sodium-doped Fe-Al-O oxygen carriers. Spherical particles are iron(II) hydroxide and needle-like structures are dawsonite.
Why is this interesting? Chemical looping hydrogen production is a process whereby hydrogen gas can be produced from a feedstock while capturing CO2. Fe-based oxygen carriers are used to keep this process continuous. Sodium doping was found to keep the oxygen carriers active through each hydrogen production cycle. However, it was not clear how sodium doping worked as the salts formed would dissolve during sample preparation. Traditional X-rays could not clarify the nature of the insoluble sodium salt. The team used SEM to confirm the presence of dawsonite as the source of sodium, thanks to its unique column-like crystal shape.
What happened since producing this image? Since this study, the research group has published a series of follow-up studies on understanding complex oxide systems as oxygen carriers for a variety of chemical looping applications. This includes hydrogen production via methane reforming. This image was one of the first projects to appear in our first Biannual Research Report in May 2014. The lead researcher, Asst Prof Paul Liu, left CARES in 2016. However, he became re-affiliated with CARES (now as a PI) when he started his tenure-track assistant professor position at NTU in 2018.
Acknowledgements Asst Prof Paul Liu performed this research under the supervision of Prof John Dennis (past-CARES Governing Board member). This image was taken at the Department of Materials Science and Metallurgy at the University of Cambridge. Financial support came from NRF* and the Engineering and Physical Sciences Research Council.
5. Translating alien linguistics

The Centre for Lifelong Learning and Individualised Cognition (CLIC) Structure Learning Task involves research participants using learned patterns to anticipate the upcoming visual symbol in a sequence.
Why is this interesting? Structure Learning (SL) refers to the brain’s ability to adaptively reorganise its neural pathways and connections to better process and respond to new information or changing environments. Research participants are tasked to seek patterns when presented with random stimuli. The ability of “patterning” encourages rule exploration as opposed to memorisation. CLIC believes SL is a building block for cognitive flexibility. The aim is to characterise the relationship between cognitive flexibility with academic outcomes, creativity, problem-solving, and socio-emotional skills in Singaporean adolescents and young adults.
What happened since producing this image? The research team is completing data analysis collected from approximately 400 secondary school students and 400 young adults over the last 2.5 years in Singapore. Once data analysis is complete, the team aims to perform necessary modifications to the Structure Learning Task to optimise the learner’s needs and as a fundamental training approach for cognitive flexibility.
Acknowledgements This research was conducted under the guidance of NTU PIs Profs Annabel Chen, Victoria Leong, Georgios Christopoulos, Balazs Zoltan Gulyas, David Hung, and Dr Teo Chew Lee. Cambridge Pis include Profs Zoe Kourtzi, Michelle Ellefson, John Suckling, Henriette Hendriks and Senior Scientific Advisors Profs Barbara Sahakian and Trevor Robbins. Financial support came from NRF*.
6. A golden opportunity
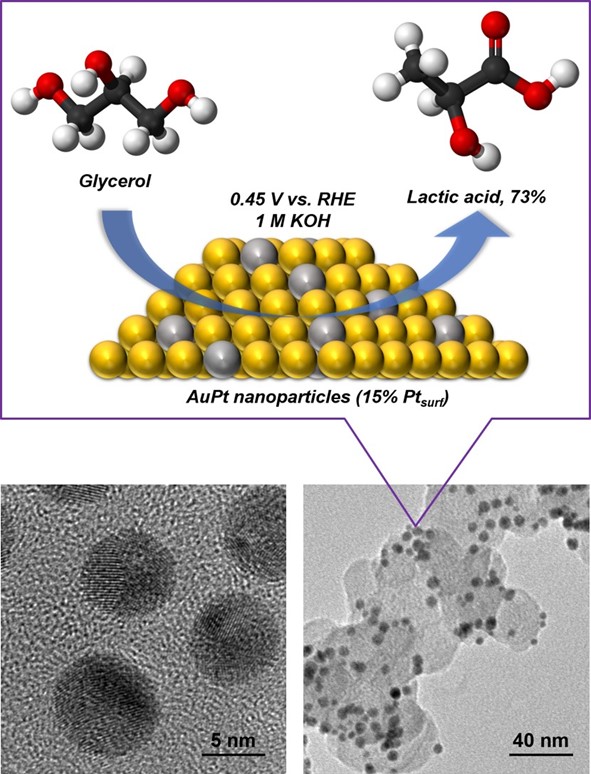
CARES researchers discovered that gold-platinum nanoparticles can directly produce lactic acid from electrochemical glycerol oxidation at room temperature and pressure. This important compound is a versatile additive in many segments of the chemical industry.
Why is this interesting? This is the first time scientists have synthesised lactic acid from glycerol via electrochemical methods. This is a highly efficient and environmentally friendly way to produce valuable chemicals from relatively inexpensive feedstocks. CARES researchers prepared gold-platinum bimetallic nanoparticles with various surface compositions to study and optimise the production of lactic acid. The optimised catalyst dramatically improved the lactic acid molar selectivity and decreased the electricity consumption for coupling hydrogen production compared with traditional oxygen evolution reactions.
What happened since producing this image? Since this breakthrough, the researchers have published a series of papers on the electrochemical production of high-value chemicals from relatively inexpensive feedstocks as follow-up studies. They hope to bring this discovery from the lab to industry by applying the catalysts in a membrane-electrolyte-assembly electrolyser to produce hydrogen at industrial-level current densities.
Acknowledgements This work was carried out by Dr Chencheng Dai and Dr Libo Sun at CARES and guided by Prof Adrian Fisher and Prof Zhichuan Xu. Financial support came from NRF*.
7. From emissions to solutions
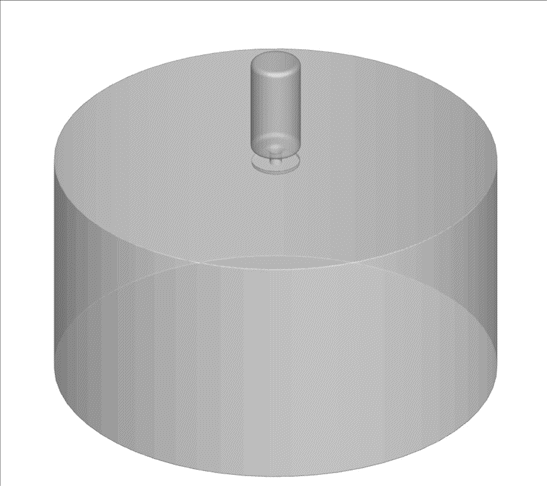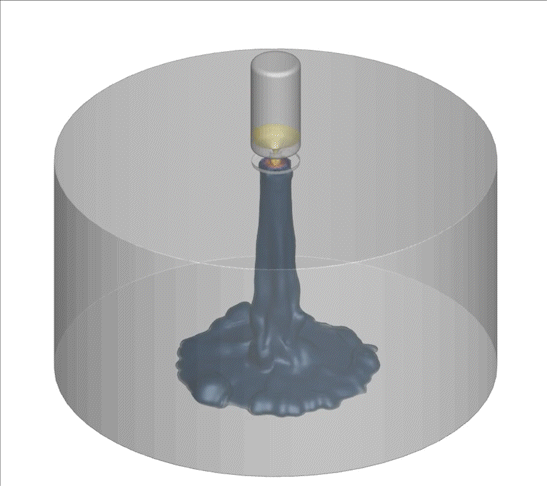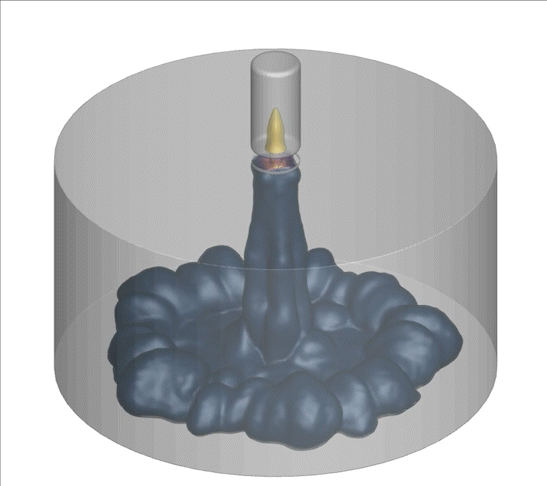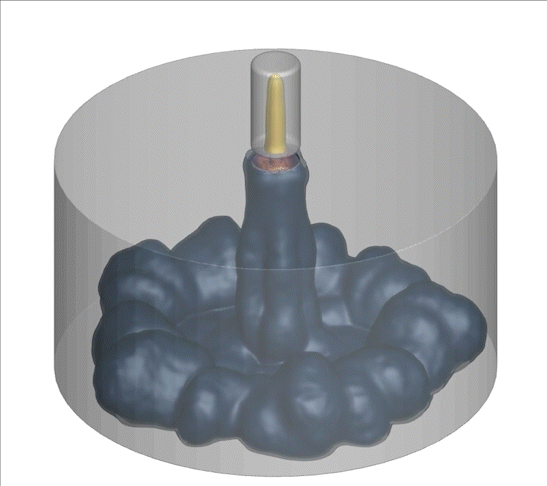
A computational technique used to model random and complex systems was performed on an advanced internal combustion system called turbulent jet ignition. The system consumed a lean fuel mixture of methane-air in the main chamber; the pre-chamber is spark ignited with a highly flammable fuel.
Why is this interesting? Low/zero carbon fuels are harder to ignite using traditional methods. Additionally, the combustion of these fuels using lean combustion such as the turbulent jet ignition method can improve thermal efficiency, fuel economy, and lower emissions. There is a need to model the complex turbulence-chemistry interactions in these new technologies to evaluate their emissions. CARES researchers are using a computational technique called doubly-conditioned moment closure coupled with large eddy simulations (DCMC-LES) which can study these complex industrial applications with detailed chemistry at lower computational cost.
What happened since producing this image? LES-DCMC will be expanded to include adaptive mesh capability which will further its application in other industrial and research applications. One example of LES-DCMC’s use-case in future challenges is for investigating dual-fuel systems which are gaining attention as they help in reducing emissions. They are also versatile for many applications, ranging from jet engines to gas turbines for power generation, and will aid in zero-carbon goals.
Acknowledgements Dr B Harikrishnan worked with Dr Savvas Gkantonas from the Department of Engineering, University of Cambridge, to develop the chemistry solver, guided by Prof Epaminondas Mastorakos. Financial support came from NRF*.
8. A search engine for Singapore’s land use
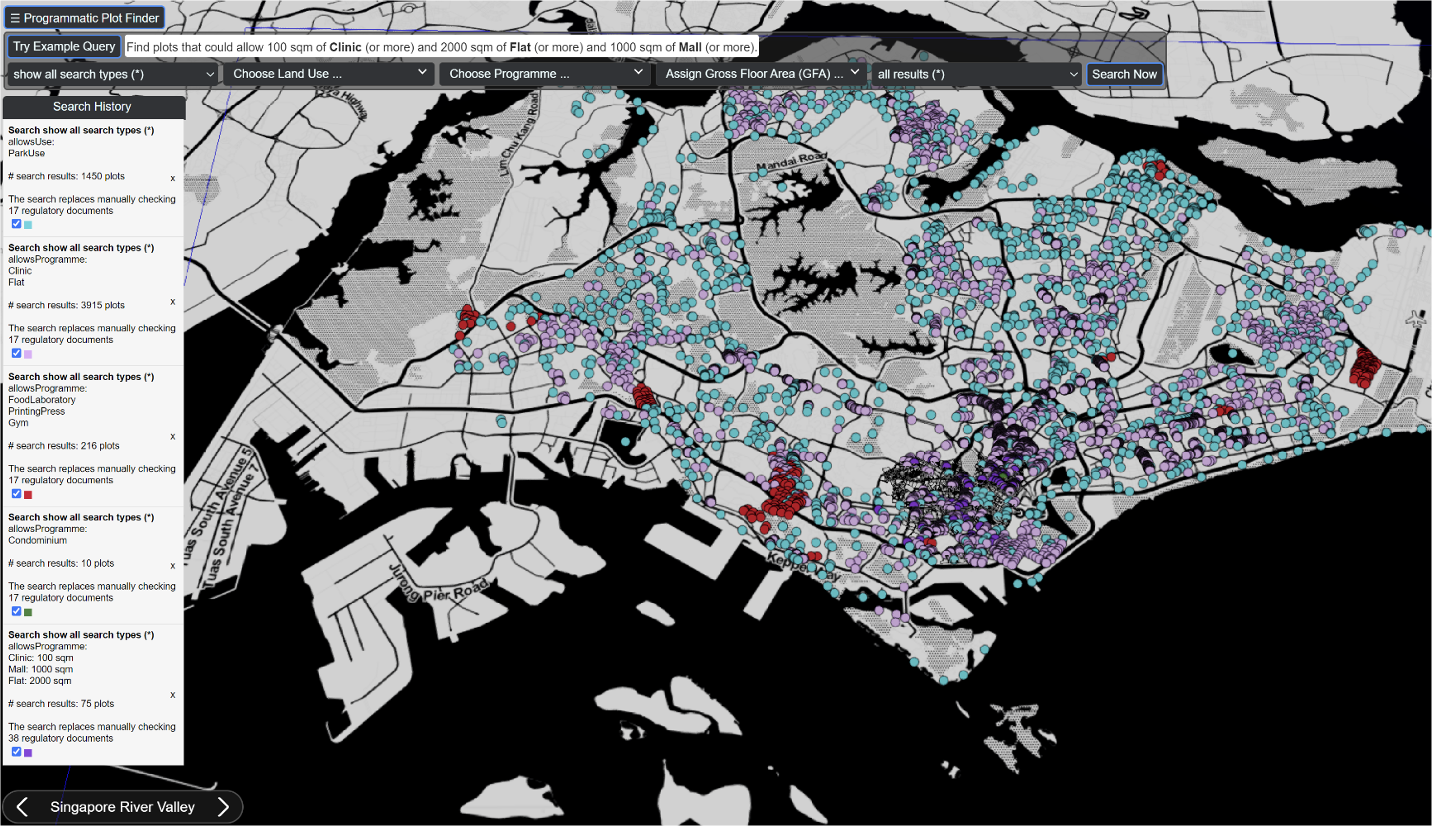
The Programmatic Plot Finder (PPF) application, is a web-based search engine that can find all plots in Singapore allowing particular combinations of land uses or programmes, including specifying minimum allowable Gross Floor Areas per programme. A single search using the PPF can replace close to 1.2 million human verifications of regulation documents.
Why is this interesting? The PPF automates large parts of city planning use cases and replaces manually consulting, verifying and processing multiple regulatory documents per plot considered for a particular use.
A single query using the PPF search engine replaces up to 1,175,136 human verifications of regulation documents. The same knowledge graph can be used to analyse geospatial characteristics of planning regulations, quantify regulatory complexity or development certainty, or assess impacts of regulatory changes (e.g. Master Plan updates), among other use cases.
What happened since producing this image? The researchers have added a second application, the Suitable Site Selector, to complement this search engine. The Suitable Site Selector lets users compare up to five plots, as well as their 500m contexts (a ‘7.5minute city’), integrating various information about present and potential future conditions of the plots and their contexts.
Acknowledgements Cities Knowledge Graph is an Intra-CREATE collaborative project involving CARES and the Singapore-ETH Centre, which is ETH Zurich’s presence in Singapore. Application development was a collaborative effort by many researchers of the CKG team, with SEC focusing on application design, ontology development, front-end web development, and computational design, building on CARES knowledge graph architecture. Financial support came from NRF*
9. The roadmap of carbon dioxide conversion
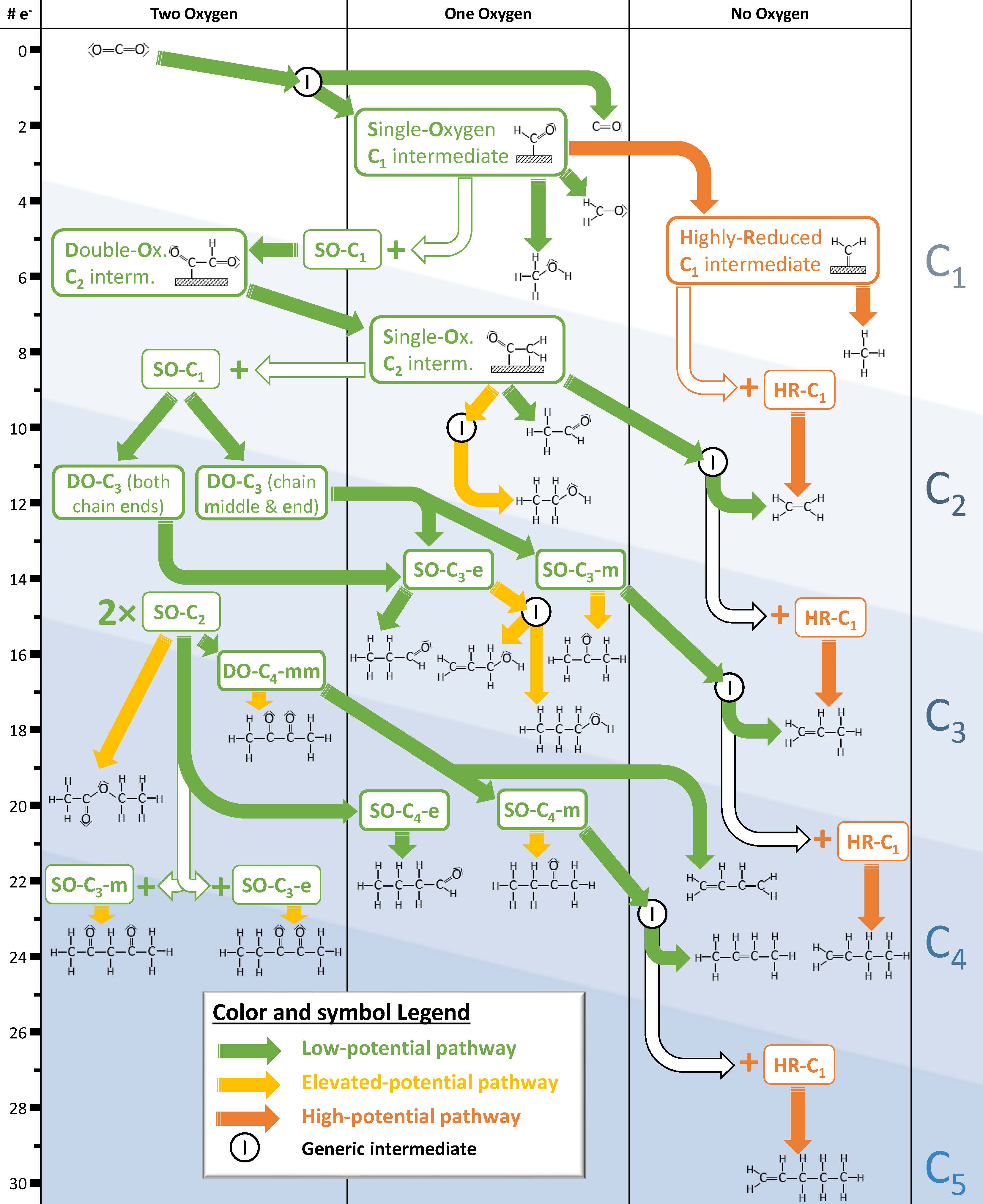
A map of products obtained from the electrocatalytic reduction of CO2. The study identified the likely key intermediates for each product and their reaction pathways.
Why is this interesting? Electrocatalytic CO2 reduction is a process that uses an electrocatalyst to convert CO2 into valuable carbon-based compounds like fuels and commodity chemicals. Increasing the conversion efficiency is the critical barrier for this process to be economically feasible. The researchers identified more than 20 products from the reaction, some previously unknown, including C5 species. They found two different reaction pathways for selectivity: one creates double bonds in the middle of carbon chains at low energy levels, and one creates double bonds at the end of chains at higher energy levels.
What happened since producing this image? The research has contributed to a deeper understanding of the mechanisms involved in the catalytic electroreduction of CO2 and encourages the scientific community to engage in open discussions on these discoveries. Furthermore, it paves the way for future endeavours aimed at developing more stable and durable catalysts, as well as enhancing selectivity towards specific products.
Acknowledgements eCO2EP was an Intra-CREATE project involving CARES and BEARS, the University of Berkeley’s presence in the CREATE Tower. This research was conducted by Simon Rihm, Dr Mikhail Kovalev, Prof Alexei Lapkin, Prof Joel Ager, and Prof Markus Kraft. Financial support came from NRF*.
10. Exploring the chemical space

A reaction network containing 39,000 chemical reactions (purple nodes) and 28,000 chemicals (red nodes). Each node in the network has a link from the reactant to the generated product. The size of the red nodes represents chemicals that are more popular as reactants and products in reactions.
Why is this interesting? In the era of big data, we have reaction databases such as Reaxys® containing massive amounts of reaction data. The researchers explored the chemical ‘space’ and discovered optimal pathways from reactants to products for sustainability metrics that can be easily pinpointed. As the chemical value chain seeks to decarbonise, there is a drive towards using biomass and biowaste ( e.g., oil palm residue) as raw materials. The reaction network here reflects some of the most important value-added chemicals (in red) that can be targeted based on how well-connected they are.
What happened since producing this image? Research is ongoing to integrate such large reaction networks to help plan sustainable routes from a target chemical product to its raw materials. Large knowledge graphs are also being developed to further augment reaction networks. Chemical Data Intelligence Pte. Ltd. (CDI), a spin-off from CARES, is investing in the development of cutting-edge software tools such as these to systematically harness sustainable processes. This will help to transform the existing fossil fuelbased carbon industry into a more sustainable one.
Acknowledgements PhD student Adarsh Arun, Dr Zhen Guo, and Prof Alexei Lapkin were involved in the generation of this image. Dr Guo and Prof Lapkin are co-founders of CDI which was set up to commercially exploit chemical data networks. Financial support came from NRF*. Technical support was provided by RELX Intellectual Properties SA which enabled mining Reaxys®. The underlying reaction data was made accessible via the Elsevier R&D Collaboration Network.
*NRF is short for the National Research Foundation, Prime Minister’s Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) programme.
Reaxys® is a trademark of Elsevier Limited.


