ELECTROSYNTHETIC PATHWAYS
for advanced low-carbon chemical manufacturing
In IRP2, low carbon electrosynthetic processes and technologies are developed which target local, on-scale and on-demand conversion of electricity to commodity or specialty chemicals.
As the contribution of renewables to the total electricity generation capacity continues to grow, novel technological opportunities arise for direct chemical conversion of the newly available low carbon electrons.
This project addresses core challenges to the implementation of low carbon, on-demand driven advanced manufacturing of chemical targets via electrosynthesis.
FEATURED RESEARCH
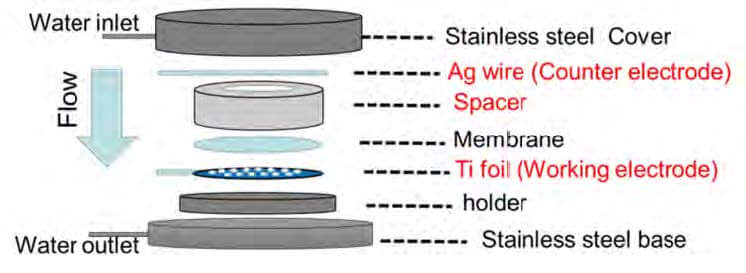
Electrolytic synthesis reactor engineering
Fouling is a challenge for membrane applications in seawater treatment. A new electrode-integrated device was designed to overcome the specific issues of membrane salt rejection performance and water flux. The device uses porous titanium foil as the working electrode, which is placed at the back of membrane. A silver wire with a diameter of 0.25 mm is used as the counter electrode and is positioned above the membrane, while being separated from the latter with a polypropylene plastic spacer. The water flux through the membrane can then be unaffected. More importantly, with this design the salt rejection of the electrode-integrated membrane in reverse osmosis (RO) application is ~90% and comparable to a membrane without the electrode.
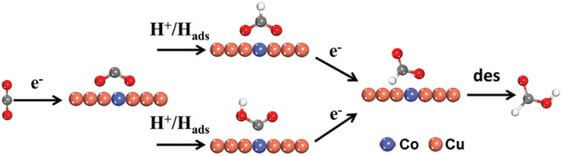
Electroreduction of carbon dioxide to formic acid
The development of highly efficient, selective, and economic approaches for electrochemical reduction of carbon dioxide to hydrocarbons is a promising way to promote the sustainable carbon cycle. A stable cobalt‐decorated copper catalyst has been developed with significantly enhanced selectivity toward formic acid produced from CO2 through electrochemical reduction. This catalyst is prepared through the electrodeposition of cobalt on the surface of copper, followed by Ar and air atmosphere treatment.
